Clean Room Systems Egypt Factory 2026
Clean Room Systems have become foundational infrastructure in pharmaceutical and industrial manufacturing rather than optional enhancements. In regulated environments—particularly sterile pharmaceutical manufacturing—any lapse in environmental control directly threatens product quality, regulatory standing, and operational continuity. At Shabab tec, this reality shapes how clean room solutions are approached, engineered, and delivered, with a clear focus on operational risk rather than theoretical design targets.
This guide delivers a comprehensive, practice-oriented view of Clean Room Systems as they are actually designed, deployed, and operated in pharmaceutical and industrial facilities. The emphasis is on real operating conditions, not abstract compliance models, reflecting the challenges faced by manufacturers in markets such as Egypt. Cost pressure, regulatory scrutiny, energy efficiency, and system reliability must be balanced continuously—an environment where Shabab tec experience in local and regional projects becomes directly relevant.
Clean rooms are treated here as integrated systems, not isolated spaces. HVAC, air filtration, monitoring, partitioning, and operational workflows function as a single ecosystem, where weak integration often poses greater risk than individual component limitations. This system-level perspective mirrors how Shabab tec structures clean room projects in practice, supporting engineering teams, quality departments, facility managers, and decision makers across planning, procurement, implementation, and long-term operation.
What Is a Clean Room System
A Clean Room System is a controlled environment engineered to limit contamination from airborne particles, microorganisms, temperature variation, humidity fluctuation, and pressure imbalance. The defining characteristic is systemic control. A clean room does not function as a standalone room but as a coordinated set of interdependent elements.
In operational terms, a clean room system includes:
- Clean room HVAC system
- Clean room air filtration system
- Environmental and particle monitoring
- Physical infrastructure such as partitioning systems and pass-throughs
- Operational procedures, validation, and ongoing control
Within pharmaceutical production—and particularly in sterile pharmaceutical manufacturing (التصنيع الدوائي المعقم)—these systems protect product integrity from raw material handling through final packaging, where risk exposure is cumulative and unforgiving.

Why Clean Room Systems Are Critical in Pharmaceutical Manufacturing
Pharmaceutical products respond sharply to environmental instability. Minor deviations in air quality, pressure differentials, or particulate levels can lead to:
- Product contamination
- Batch rejection
- Regulatory non-compliance
- Unplanned production stoppages
Clean Room Systems establish controlled conditions that support sterility assurance, consistent product quality, regulatory alignment, and protection of both products and personnel.
In markets like Egypt, manufacturers often face a narrow margin between cost efficiency and compliance risk. Properly designed clean room systems reduce long-term exposure by stabilizing operations and limiting corrective actions, which are typically far more expensive than preventive control.
Clean Room Systems vs Conventional Controlled Environments
A common misconception is that conventional air conditioning combined with basic filtration provides sufficient control for sensitive pharmaceutical operations. In practice, conventional controlled environments lack several capabilities that define Clean Room Systems.
Key distinctions include:
- Airflow control: Clean Room Systems manage airflow direction and velocity, not just thermal conditions.
- Filtration efficiency: HEPA-based systems remove particle sizes beyond the capability of standard filtration.
- Pressure zoning: Pressure differentials are actively maintained to prevent cross-contamination.
- Monitoring: Continuous monitoring verifies control rather than assuming it.
This difference becomes critical when facilities expand existing production lines or retrofit older pharmaceutical plants without revisiting environmental control strategy.
Problems Clean Room Systems Are Designed to Solve
Contamination Risks in Sterile Pharmaceutical Environments
Contamination remains one of the dominant operational risks in sterile pharmaceutical manufacturing. Common sources include personnel movement, raw materials, equipment interfaces, and airborne particles or microorganisms.
Without an integrated clean room system, these risks are managed inconsistently. Clean room HVAC systems combined with air filtration and pressure control materially reduce contamination probability. In practice, many failures trace back not to individual component quality, but to weak integration and limited visibility into system performance.
Regulatory Compliance Challenges
Regulatory oversight extends across the entire lifecycle of a pharmaceutical facility. Compliance is sustained through operation, not achieved at commissioning.
Recurring challenges include:
- Maintaining stable environmental conditions
- Generating defensible performance records
- Managing deviations and audit responses
- Closing the gap between written procedures and actual behavior
Clean room monitoring systems address these pressures directly. Continuous monitoring (المراقبة المستمرة) enables early detection of deviations, supports corrective action, and provides documented evidence of control that withstands inspection.
Operational Inefficiencies Without Integrated Systems
When clean room components are treated as independent assets rather than as a single system, inefficiencies compound:
- Excessive energy consumption from poorly coordinated HVAC operation
- Maintenance-driven downtime
- Limited flexibility when production demand increases
- Escalating long-term operating costs
An integrated clean room system aligns design intent with operational reality. For facilities planning expansion or product diversification, this alignment is not optional.

Core Components of Clean Room Systems
Clean Room Systems rely on continuous, measurable, and predictable environmental control. Each component has a defined role, but system performance depends on interaction, not individual specifications.
Clean room HVAC system
Clean Room HVAC System
The clean room HVAC system forms the backbone of environmental control. Unlike conventional HVAC, it manages airflow patterns, pressure differentials, and contamination levels in parallel with temperature and humidity.
Core functions include:
- Supplying filtered air at controlled velocities
- Maintaining stable temperature and humidity ranges
- Establishing positive or negative pressure zones حسب طبيعة العملية
- Supporting uninterrupted operation without instability
HVAC Clean Room System Design Principles
Effective HVAC clean room system design begins with process understanding rather than floor area calculations. Key considerations include air change rates aligned with room classification, airflow strategy selection, pressure cascades between rooms, and redundancy to maintain continuity.
In Egyptian pharmaceutical facilities, difficulties often arise when imported design concepts are applied without adapting to local climate, energy pricing, or maintenance capacity.
Clean Room Air Conditioning System
The air conditioning function within a clean room must support personnel comfort without destabilizing airflow or particulate control. Rapid temperature swings disrupt airflow behavior and undermine classification stability.
A functional clean room air conditioning system balances operator comfort, energy efficiency, and process stability, accepting that compromise is unavoidable but misalignment is not.
Clean Room Air Filtration System
Air filtration sits at the center of contamination control. Clean room air filtration systems typically depend on HEPA filters to remove particles capable of compromising sterility.
Critical factors include filter efficiency, placement, air distribution uniformity, integrity testing, and lifecycle management. In practice, filtration underperformance is more often linked to installation errors or inconsistent maintenance than to filter specification alone.
Clean Room Monitoring System
A clean room monitoring system provides continuous visibility into environmental conditions and performance trends, supporting both operational control and regulatory compliance.
Clean Room Continuous Monitoring System
Continuous monitoring (نظام المراقبة المستمرة) tracks particle counts, temperature, humidity, differential pressure, and airflow velocity. Early deviation detection allows intervention before product quality is affected.
Particle Monitoring and Environmental Control
Particle monitoring systems are particularly critical in sterile pharmaceutical manufacturing. They support clean room classification, validation and requalification activities, and audit-ready documentation. Increasingly, these systems are expected to integrate with facility management and quality platforms rather than operate in isolation.
Clean Room Communication System
Communication within clean room environments must preserve contamination control while supporting coordination. Clean room communication systems reduce unnecessary movement and door openings through intercoms, visual indicators, and integrated alarms. Their contribution to contamination risk reduction is operational rather than theoretical.
Clean Room System Types by Application
Clean Room System in Sterile Pharmaceutical Manufacturing
Sterile pharmaceutical manufacturing demands the highest level of environmental control. Clean room systems in this context support aseptic processing, sterile filling, and controlled personnel and material flow. Reliability, redundancy, and strict monitoring take priority over simplicity.
Clean Room System for Pharmaceutical Industry Facilities
Not all pharmaceutical processes require identical control levels. Clean room systems are adapted based on dosage form, process sensitivity, and regulatory expectations, allowing manufacturers to allocate investment where environmental risk is highest.
Clean Room System of Pharmacy Design
In pharmacy design (تصميم الصيدليات التحضيرية), clean room systems focus on controlled compounding, operator and patient protection, and efficient workflows within constrained spaces. Ease of operation often carries equal weight to technical performance.
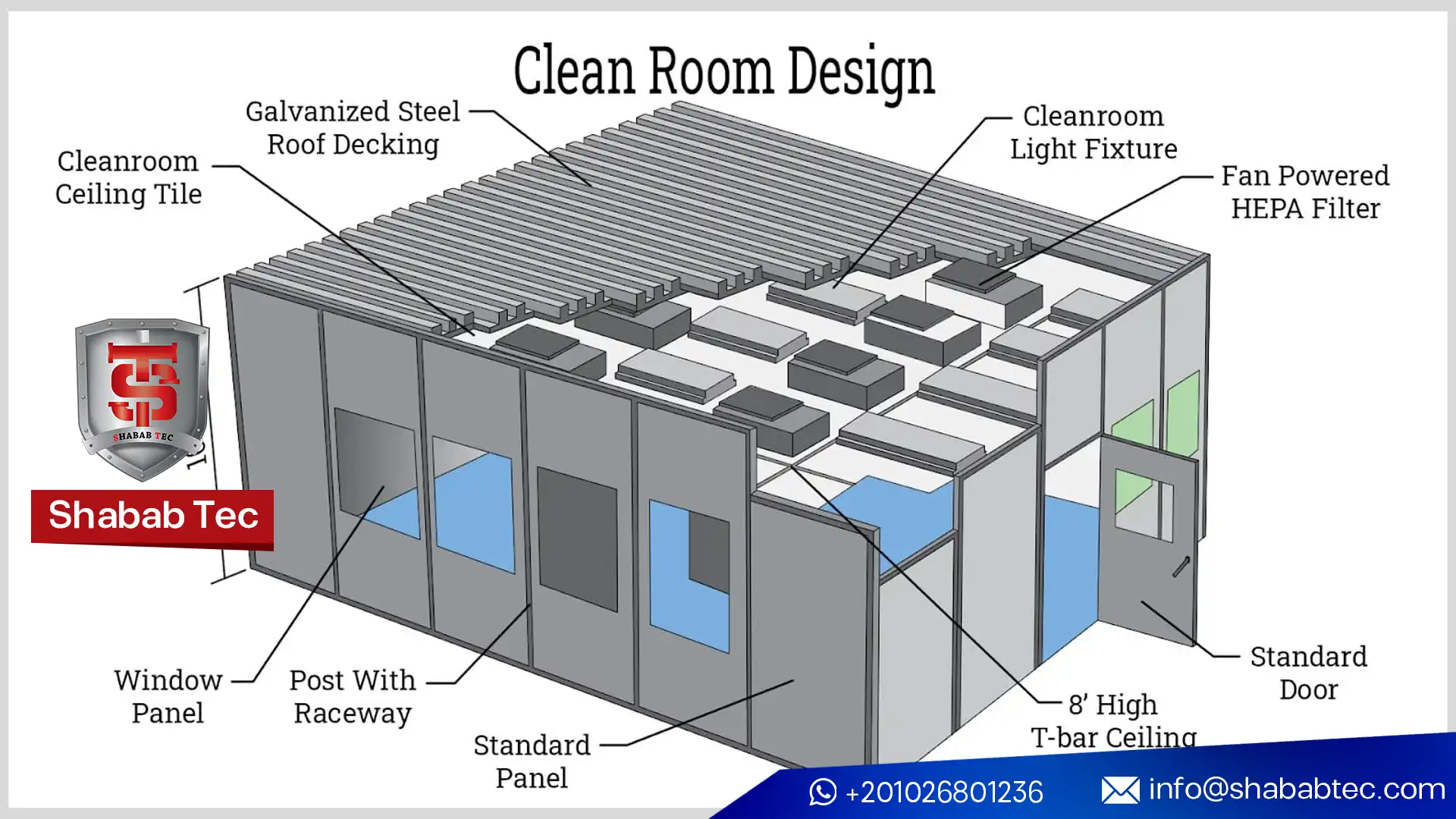
Clean Room Design, Layout, and Partitioning
Clean Room Partitioning Systems
Partitioning systems define clean room boundaries and enable controlled airflow. Modern clean room partitioning systems are modular, cleanable, and compatible with HVAC and monitoring infrastructure, allowing reconfiguration as production needs change.
Clean Room Pass Through System
Clean room pass through systems enable material transfer while preserving pressure balance. They reduce personnel movement, limit cross-contamination, and support efficient workflows. Design must align precisely with room classification and process risk.
Workflow Optimization in Pharmaceutical Clean Rooms
Layout decisions directly influence contamination risk, productivity, and operating cost. Poor workflow design introduces bottlenecks and compliance exposure. Optimized workflows support scalability and regulatory stability, particularly for manufacturers planning long-term growth.
Standards, Compliance, and Industry Expectations
Clean Room Systems are evaluated by sustained performance, not design intent. In pharmaceutical manufacturing, compliance is operational and continuous.
Clean Room Classification and Environmental Requirements
Clean room classification defines acceptable particle and environmental limits, driving HVAC design, filtration levels, monitoring frequency, and operating procedures. In sterile environments, even short deviations can trigger investigation or shutdown.
Facilities in Egypt commonly align internal standards with international expectations to maintain export readiness and inspection resilience.
Validation, Monitoring, and Documentation
Validation without continuous verification is incomplete. Clean room systems must demonstrate repeatable performance, controlled parameters, and traceable historical data. Monitoring systems form the data backbone for audit readiness, deviation response, and risk reduction. Documentation quality often separates stable operations from recurring regulatory exposure.
Market Expectations vs Regulatory Reality
Budget constraints, energy costs, and workforce capability frequently collide with regulatory expectations. Sustainable clean room system strategies account for long-term operating cost, maintenance capacity, and availability of local technical support, prioritizing durability over short-term compliance fixes.
Comparing Clean Room System Approaches
Integrated vs Modular Clean Room Systems
Integrated systems deliver performance consistency, centralized monitoring, and long-term stability. Modular systems offer faster deployment, scalability, and lower initial investment. The appropriate choice depends on process complexity, growth trajectory, and regulatory exposure.
Manual vs Automated Monitoring Systems
Manual monitoring introduces human error, limited data resolution, and delayed response. Automated clean room monitoring systems improve accuracy, response speed, and documentation integrity. Automation increasingly reflects baseline expectation rather than premium capability.
Return on Investment (ROI) Considerations
ROI extends beyond capital cost. Energy efficiency, downtime reduction, maintenance intensity, and compliance risk mitigation determine total cost of ownership. Well-designed clean room systems stabilize output while reducing cumulative operational cost.
Real-World Applications and Case Perspectives
Case for How Companies Apply Clean Room Systems
Pharmaceutical companies often deploy clean room systems incrementally, prioritizing high-risk processes before expanding to supporting areas and integrating automation. This phased approach aligns investment with operational maturity.
Pharmaceutical Industry Use Case Overview
In sterile pharmaceutical manufacturing, clean room systems support aseptic filling, controlled transfer, and personnel flow management. Success depends on aligning system design with daily operating behavior rather than theoretical models.
Future of Clean Room Systems and Emerging Innovations
Automation, Robotics, and AI Integration
Automation increasingly shapes clean room operations through robotic material handling, AI-driven analytics, and predictive maintenance. Reduced human intervention lowers contamination risk and improves consistency.
Sustainable and Energy-Efficient Designs
Energy efficiency has moved from cost concern to strategic priority. Modern clean room systems emphasize airflow optimization, energy recovery, and intelligent controls, supporting both operational efficiency and corporate responsibility.
How to Evaluate and Select the Right Clean Room System
Technical Criteria
Evaluation begins with classification requirements, HVAC and filtration performance, and monitoring capability. Systems must be engineered for reliability under real operating conditions.
Operational Criteria
Operational viability depends on maintainability, usability, and training burden. Systems that are technically advanced but operationally complex often fail over time.
Long-Term Reliability and Support
Local technical support and spare parts access are decisive, particularly where downtime disrupts supply commitments. Selecting a clean room system partner carries as much weight as selecting the system itself.
Frequently Asked Questions
What is the difference between a clean room system and standard HVAC?
Clean room systems control particles, pressure, and contamination in addition to temperature.
Why is continuous monitoring important in pharmaceutical clean rooms?
It enables early deviation detection and supports defensible compliance documentation.
Are modular clean room systems suitable for sterile pharmaceutical production?
They can be, provided design and validation match application risk.
How often should clean room systems be revalidated?
Revalidation depends on risk level, regulatory expectations, and performance history.
Do clean room systems need customization for local conditions?
Yes. Climate, energy costs, and operational practices materially affect performance.
What factors most affect clean room system operating costs?
Energy consumption, maintenance frequency, and monitoring integration.
Next Steps….
Clean Room Systems represent long-term commitments to product quality, compliance stability, and operational resilience. Facilities that succeed treat clean rooms as integrated systems aligned with business priorities, not isolated engineering projects.
For organizations planning new facilities, upgrading existing clean rooms, or reassessing HVAC and monitoring performance, the next step is a focused technical and operational assessment grounded in actual production requirements rather than assumptions.






