Clean Room System Pass Boxes
Clean Room System Pass Boxes – Static & Dynamic Solutions for Pharmaceutical Classified Areas
Pass boxes are essential components in any clean room system, specifically designed to control material transfer between areas of different cleanliness classifications. In pharmaceutical facilities, pass boxes play a critical role in preventing cross-contamination while maintaining compliance with GMP guidelines.
Shababtec designs and manufactures clean room system pass boxes in both static and dynamic configurations, engineered for use in pharmaceutical classified areas, laboratories, and controlled manufacturing environments.
What Is a Pass Box in a Clean Room System?
A pass box is a sealed enclosure installed within clean room walls to allow the transfer of materials between two areas without direct personnel movement. This controlled transfer minimizes airborne contamination and supports clean room zoning.
Pass boxes are commonly installed between:
- Clean and non-clean areas
- Different classified clean room zones
- Production and packaging rooms
In pharmaceutical applications, pass boxes must be designed in accordance with pass boxes in classified areas pharmaceutical guidelines.
Clean Room System Pass Boxes – Static Type
Clean room system pass boxes static are designed for material transfer between areas with the same or similar cleanliness classification.
Static Pass Box Characteristics
- No active air circulation
- Mechanical or electromagnetic interlock system
- Smooth internal surfaces for easy cleaning
- Flush integration with clean room wall panels
Static pass boxes are widely used in pharmaceutical facilities where controlled but simple material transfer is required.

Clean Room System Pass Boxes – Dynamic Type
Clean room system pass boxes dynamic are used when materials are transferred between areas of different cleanliness classifications.
Dynamic pass boxes are equipped with active air handling systems to reduce particle contamination during transfer.
Dynamic Pass Box Functional Features
- Integrated air circulation system
- HEPA filtration for particle removal
- Positive pressure inside the chamber
- Interlock system preventing simultaneous door opening
These systems are commonly specified as dynamic pass box pharmaceuticals.
Dynamic Pass Box for Pharmaceutical Applications
In pharmaceutical manufacturing, dynamic pass boxes are critical for maintaining contamination control between classified areas.
- Used between Grade C to Grade B areas
- Used between preparation and filling zones
- Supports GMP environmental control strategies
Dynamic pass boxes help maintain pressure differentials and reduce contamination risk during material transfer.
Pass Boxes in Classified Areas – Pharmaceutical Guidelines
Pharmaceutical guidelines require that pass boxes installed in classified areas must:
- Prevent simultaneous door opening
- Maintain clean room pressure balance
- Have smooth, cleanable internal surfaces
- Be easy to disinfect and maintain
Shababtec pass boxes are engineered to meet these pharmaceutical requirements while integrating seamlessly with modular clean room systems.

Pharmaceutical Pass Box with UV Light
For additional surface decontamination, pharmaceutical pass boxes can be equipped with UV light systems.
- UV light for surface microbial reduction
- Integrated safety interlock
- Used for sensitive material transfer
UV-equipped pass boxes are commonly used in pharmaceutical laboratories and sterile preparation areas.
Pass Box Dimensions & Customization
Pass box dimensions are selected based on material size, workflow requirements, and wall thickness.
Shababtec manufactures pass boxes with:
- Standard dimensions for common applications
- Custom dimensions for project-specific needs
Dynamic systems are often specified using dynamic pass box dimension requirements defined during engineering design.
Pharmaceutical Dynamic Pass Box Drawing
For pharmaceutical projects, dynamic pass boxes are supported by detailed technical drawings showing:
- Overall dimensions
- Door interlock configuration
- HEPA filter placement
- Airflow direction
These drawings support engineering coordination, validation, and installation accuracy.
Static vs Dynamic Pass Boxes in Clean Room Systems
Selecting between clean room system pass boxes static and clean room system pass boxes dynamic is a critical engineering decision in pharmaceutical facilities. The wrong selection can compromise contamination control and regulatory compliance.
When to Use Static Pass Boxes
Static pass boxes are suitable when material transfer occurs between areas with the same or closely related cleanliness classification.
- Transfer between two Grade D areas
- Transfer within the same production zone
- Low-risk material movement
In these cases, a static system provides controlled transfer without the complexity of active air circulation.
When to Use Dynamic Pass Boxes
Dynamic pass boxes are required when material transfer occurs between areas of different cleanliness classifications.
- Transfer from unclassified to classified areas
- Transfer between Grade C and Grade B rooms
- High-risk pharmaceutical processes
This configuration is commonly specified as dynamic pass box pharmaceuticals.

Dynamic Pass Box Operation in Pharmaceutical Facilities
Dynamic pass boxes operate using a controlled airflow system that actively removes airborne particles during material transfer.
Airflow Principle
- Air is drawn through a pre-filter
- Air passes through a HEPA filter
- Filtered air creates positive pressure inside the chamber
- Particles are flushed out before door opening
This process significantly reduces the risk of contamination when transferring materials into higher classified areas.
Dynamic Pass Box Dimensions – Engineering Considerations
Dynamic pass box dimension selection is not arbitrary. Dimensions are defined based on operational workflow and clean room wall thickness.
Factors Affecting Pass Box Dimensions
- Size of materials or containers
- Frequency of material transfer
- Available wall space
- Airflow and HEPA filter size
Oversized pass boxes increase contamination risk, while undersized units reduce operational efficiency.
Standard Pass Box Dimensions
While many pharmaceutical projects use standard pass box sizes, custom dimensions are often required.
- Small pass boxes for tools and documents
- Medium pass boxes for raw materials
- Large pass boxes for containers and trays
Shababtec manufactures both standard and custom pass box dimensions based on project requirements.
Pharmaceutical Pass Box with UV Light – Practical Use
A pharmaceutical pass box with UV light is used to reduce surface microbial load on transferred materials.
UV Light Functionality
- Surface decontamination support
- Integrated safety interlock
- Timed UV exposure cycles
UV systems are supplementary and do not replace proper cleaning or airflow control.
Pass Boxes in Classified Areas – Pharmaceutical Guidelines
According to pharmaceutical guidelines, pass boxes installed in classified areas must support contamination control and operational discipline.
- Doors must be interlocked
- Internal surfaces must be smooth and non-shedding
- Design must allow effective cleaning and disinfection
- Airflow must not disrupt room pressure balance
Compliance with these principles is essential for audit readiness and GMP inspections.
Integration with Clean Room Wall Systems
Pass boxes are integrated directly into clean room wall panels to maintain flush surfaces and airtight construction.
- No protrusions into clean room space
- Sealed perimeter between pass box and wall panel
- Alignment with clean room workflow
This integration ensures consistent clean room performance and simplifies installation.
Commercial Impact of Correct Pass Box Selection
Choosing the correct pass box configuration has a direct commercial impact on pharmaceutical operations.
- Reduced contamination incidents
- Lower validation and rework costs
- Smoother regulatory audits
- Improved production efficiency
As a manufacturer, Shababtec supports clients in selecting pass box systems that match both technical and operational needs.
Common Mistakes in Selecting Pass Boxes for Pharmaceutical Clean Rooms
Incorrect selection or poor specification of pass boxes is a common cause of contamination risk in pharmaceutical clean room systems. Many facilities focus only on dimensions while overlooking operational and regulatory factors.
Frequent Selection Errors
- Using static pass boxes where dynamic systems are required
- Oversized pass box dimensions that disrupt airflow stability
- Ignoring pressure differential requirements between classified areas
- Lack of proper interlock systems
- Improper integration with clean room wall panels
These mistakes often lead to audit observations, revalidation costs, and operational inefficiencies.
Dynamic Pass Box Validation in Pharmaceutical Facilities
Dynamic pass boxes installed in pharmaceutical clean rooms must support validation and qualification requirements.
Validation-Relevant Design Features
- Defined airflow direction inside the chamber
- HEPA filter integrity accessibility
- Documented interlock logic
- Repeatable operational performance
Properly engineered dynamic systems simplify IQ, OQ, and PQ activities during clean room qualification.
Clean Room Doors Manufacturer & Supplier
Pharmaceutical Dynamic Pass Box Drawing – Engineering Importance
A detailed pharmaceutical dynamic pass box drawing is essential for coordination between clean room architecture, HVAC systems, and process equipment.
Information Included in Pass Box Drawings
- Overall and internal pass box dimensions
- Wall thickness compatibility
- Door opening direction and interlock logic
- HEPA filter location and airflow path
These drawings reduce installation errors and support regulatory documentation.
Pass Boxes in Classified Areas – Operational Discipline
In pharmaceutical classified areas, pass boxes are part of the contamination control strategy rather than simple transfer cabinets.
- Material transfer procedures must be defined
- Door opening sequence must be respected
- Cleaning and disinfection routines must be documented
Pass box design must support these operational rules rather than relying on operator behavior alone.
Dynamic Pass Box with UV Light – Compliance Considerations
A pharmaceutical pass box with UV light is commonly specified for additional surface microbial reduction.
Engineering Considerations for UV Systems
- UV exposure time must be controlled
- UV operation interlocked with door status
- UV lamps positioned to avoid shadow zones
UV light is a supplementary feature and must be integrated without compromising airflow performance.
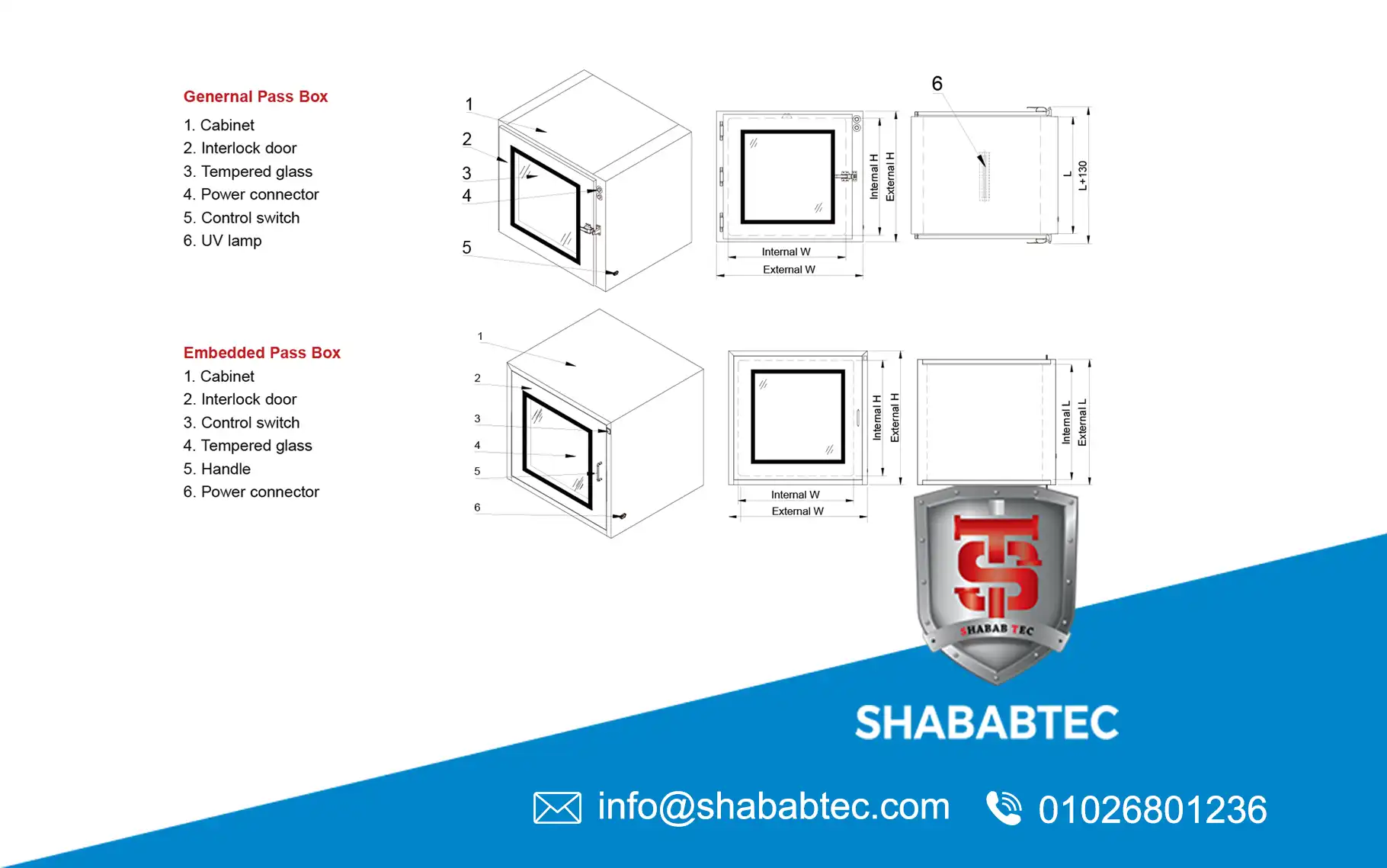
Pass Box Dimensions and Clean Room Workflow
Selecting correct pass box dimensions directly affects clean room workflow efficiency.
- Too small: material handling difficulties
- Too large: increased contamination risk
- Correct size: smooth, controlled material transfer
Dynamic systems require additional internal space to accommodate airflow components without restricting usability.
Integration of Pass Boxes within Clean Room Systems
Pass boxes must be engineered as part of the complete clean room system, not as isolated components.
- Flush installation within wall panels
- Sealed interfaces to prevent leakage
- Alignment with room pressure zoning
This integrated approach ensures stable clean room operation and long-term performance.
Commercial Impact of Proper Pass Box Engineering
From a commercial standpoint, properly engineered pass boxes reduce long-term operational costs.
- Lower contamination-related losses
- Reduced revalidation frequency
- Smoother regulatory inspections
- Improved production continuity
As a manufacturer, Shababtec supports pharmaceutical clients by delivering pass box systems engineered for both compliance and operational efficiency.
Use of Pass Boxes in Pharmaceutical Classified Areas
In pharmaceutical facilities, pass boxes are an essential element in contamination control strategies within classified areas. Their correct specification and operation directly impact product quality and regulatory compliance.
Pass boxes are commonly installed in:
- Material transfer points between unclassified and classified areas
- Transitions between Grade D, C, and B clean rooms
- Preparation and dispensing zones
Compliance with pass boxes in classified areas pharmaceutical guidelines is mandatory to ensure consistent environmental control.
Dynamic Pass Boxes in Pharmaceutical Manufacturing
Dynamic pass box pharmaceuticals applications are driven by the need to actively control airborne contamination during material transfer.
- Maintains controlled internal environment
- Reduces particle ingress into higher classified areas
- Supports clean room pressure cascade
Dynamic pass boxes are typically specified where product exposure risk is high or where regulatory scrutiny is strict.
Pharmaceutical Dynamic Pass Box Drawing & Documentation
Engineering documentation is a critical requirement in pharmaceutical projects. A detailed pharmaceutical dynamic pass box drawing supports:
- Design coordination with clean room walls and HVAC systems
- Installation accuracy
- Qualification and validation activities
Accurate drawings reduce on-site modifications and ensure alignment with GMP documentation requirements.
Pass Box Dimensions – Practical Selection Approach
Selecting correct pass box dimensions requires balancing contamination control with operational efficiency.
Dimension Selection Criteria
- Maximum size of transferred materials
- Frequency of material movement
- Wall thickness and structural constraints
- Internal airflow requirements for dynamic systems
Dynamic systems require additional internal space to accommodate airflow components without compromising usability.
Pharmaceutical Pass Box with UV Light – When to Specify
A pharmaceutical pass box with UV light is specified when surface microbial reduction is required as an additional contamination control measure.
- Used for sensitive materials and tools
- Operates with timed UV exposure cycles
- Integrated with safety interlocks
UV light systems complement airflow-based contamination control but do not replace proper cleaning or filtration.
Static and Dynamic Pass Boxes as Part of the Clean Room System
Both clean room system pass boxes static and clean room system pass boxes dynamic must be engineered as part of the overall clean room system rather than standalone units.
- Flush installation within modular wall panels
- Sealed interfaces to prevent air leakage
- Alignment with room classification strategy
This system-based approach ensures stable performance and regulatory compliance.
Clean Room Walls & Ceilings Systems
Why Pharmaceutical Facilities Choose Shababtec Pass Boxes
Pharmaceutical manufacturers require pass box systems that deliver consistent performance, audit readiness, and long-term reliability.
- Manufacturer-controlled design and fabrication
- Static and dynamic configurations available
- Custom pass box dimensions per project
- Compatibility with modular clean room wall systems
- Support for pharmaceutical classified area guidelines
Operational and Commercial Benefits
Properly engineered pass boxes deliver measurable benefits throughout the lifecycle of pharmaceutical clean room operations.
- Reduced contamination-related deviations
- Smoother regulatory inspections
- Lower revalidation and maintenance costs
- Improved material flow efficiency
Engineering Consultation for Pass Box Selection
Selecting the correct pass box configuration requires engineering evaluation based on clean room classification, material flow, and operational risk.
Shababtec provides technical consultation to define the most suitable static or dynamic pass box solution for pharmaceutical clean room systems.






